Table of Contents
- Covid-19 vaccine booster study
- Adopting a new way of working
- PHARMExcel assigned services
- Study timelines
- Added value
a. Protocol and ICD development
b. Regulatory approvals
c. Study start up procedures
d. Remote SIVs and Activation
e. Recruitment and Monitoring - Improved outcomes through collaboration and agile working
1. Covid-19 vaccine booster study
Following its reputation of excellence in delivering investigator-led studies, PHARMExcel was selected to manage the Covid-19 vaccine booster study. Funded by the Government through the National Institute for Health Research (NIHR) and the Vaccine Taskforce, the Phase II, Investigational Medicinal Product (IMP) study evaluated seven different COVID-19 vaccinations given as a third (booster) dose, recruiting participants who had previously received either two doses of BNT162b2 (Pfizer–BioNtech) or two doses of ChAdOx1 nCOV-19 (Oxford–AstraZeneca).
2. Adopting a new way of working:
All parties had been instructed that data be made available to the Joint Committee on Vaccination and Immunisation (JCVI) by the end of July 2021 to allow the Government time to analyse and decide the policy strategy for an Autumn booster programme. This meant a short window of approximately 5 months to achieve.
With this in mind, PHARMExcel realised it needed to look to an alternative way of cross-party working from the usual CRO approach to ensure critical timelines would be met. Our main end goal was to bring everyone together, working fast and efficiently, ensuring adherence to regulations, whilst maintaining the highest quality standards.
The process adopted 4 new agile strategies:
3. PHARMExcel assigned services:
PHARMExcel was informed about the study in February 2021 and asked by the Sponsor (University Hospital Southampton NHS Foundation Trust -UHS) to provide project management, monitoring, safety and quality oversight for the study, from protocol development to close-out.
The study initially involved 18 NHS sites across the UK, along with external vendors responsible for key elements of the project such as statistics, data management, central laboratories and vaccine supply.
An agile approach
Within the first week of notification, we had established;
- A core team of CRO experts including a dedicated clinical study/project manager, clinical research associates (CRA) and experienced team of clinical trial administrators (CTA). A responsibilities matrix across all parties:
- Sponsor-CRO
- Sponsor-Statisticians
- Sponsor-Data Management
- Sponsor-Vaccine Suppliers
- Sponsor-Laboratories
- Commenced protocol development across February and March 2021 with a re-design in April 2021
- Commenced site feasibility utilising the Clinical Research Network (CRN) in April 21
- Commenced background study set up-at risk- to meet aggressive timelines
As the study progressed, we also initiated a 19th site, along with four sub studies.
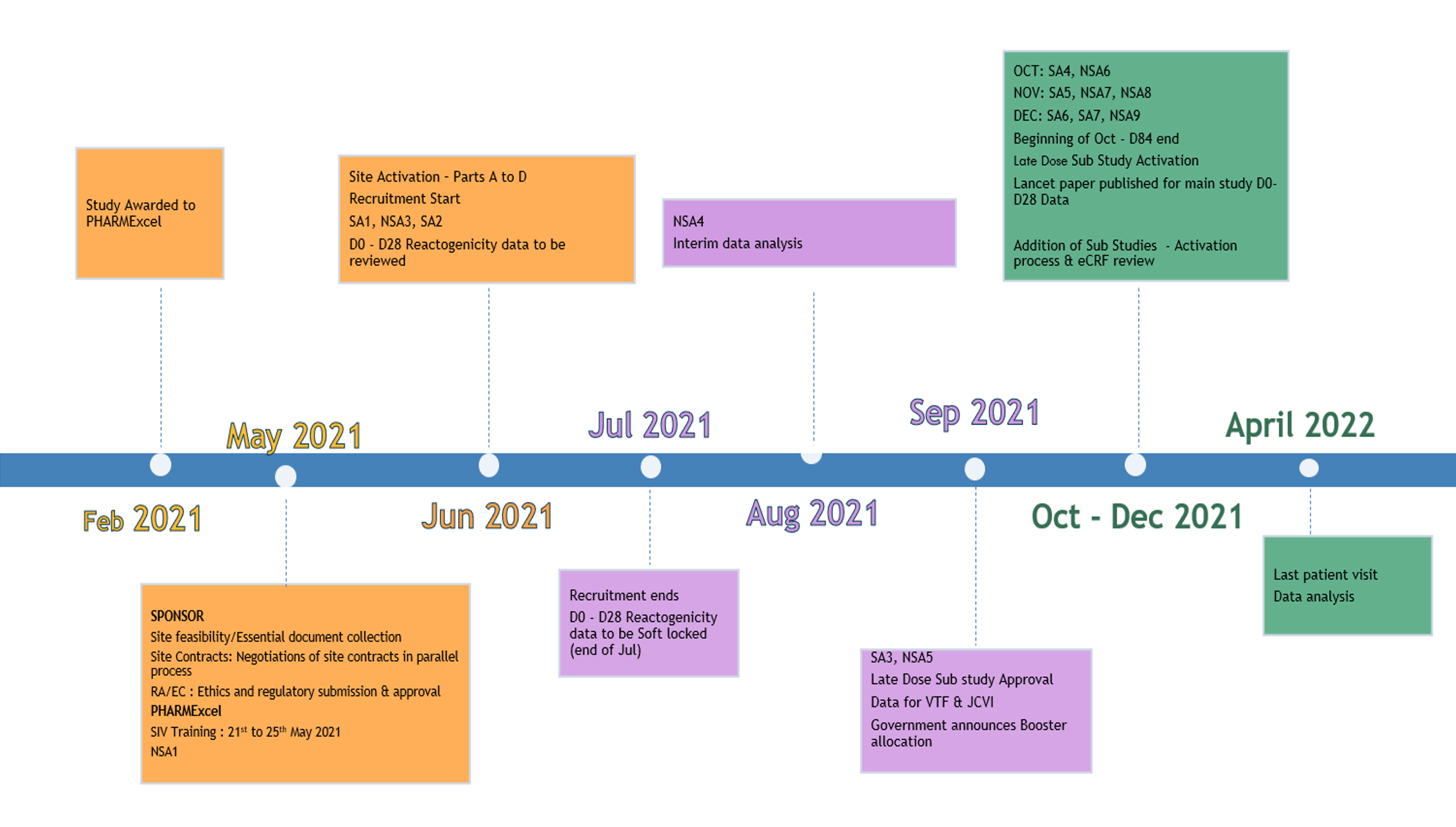
4. Study timeline
Here is an overview of the study timeline over 13 months from PHARMExcel’s (CRO) appointment on the study in February 2021 and the last patient visit data analysis in April 2022.
5. Added value
PHARMExcel was able to bring an unrivalled level of experience and leadership to the booster study gained from its previous Covid and vaccine trial work and delivery of Investigator Initiated Studies (IIS)
Moreover, having already established a long-standing partnership with the Sponsor, UHS, from multiple previous studies, we were quickly able to establish cross-party collaborative working to get the project off the ground quickly which was essential for such a critical and time sensitive study.
a. Protocol and informed consent documentation development
We provided expert and fast reviews of the key study documentation, including the clinical study protocol, the clinical study plan and informed consent documents. The team’s experience in implementing studies of this type led to significant improvements being incorporated within these documents, ensuring they were accurate, concise and in line with applicable regulations. These fast-track reviews and changes allowed a smooth and fast approval pathway for the study.
b. Regulatory approvals
The sponsor took responsibility for the Ethics, Health Research Authority (HRA) and Regulatory submissions with support from PHARMExcel in the initial stages. Rolling reviews were conducted by the EC/HRA and Medicines and Healthcare product Regulatory Agency (MHRA). We were able to submit individual documents as they were finalised, rather than as a completed document pack, which led to significant time savings. All questions were addressed from the authorities in an expedited manner, leading to approvals being granted within seven working days.
c. Study start-up procedures
Working with the sponsor and the wider study teams, the PHARMExcel team ensured the following project start-up/delivery elements were delivered to plan:
- CRO project tracking
- Development of study specific documents (i.e. site activation processes including Quality Control (QC) activities, IMP accountability documents, study specific vaccine manufacturer reporting processes)
- Efficient document management processes
- eCRF/data management review and testing
- Development of key operational documents:
- IMP Management Plan
- Quality Management Plan
- Communication Plan
- Monitoring Plan
- Safety Management Plan
- Safety reporting/management procedures, including the development of study specific SOPs and templates.
- Regular touch-base meetings with the CRA Team to ensure momentum continued.
The rapid delivery of the above project elements was only achieved through daily communications between sponsor, study sites and data management vendors. All communications resulting in decision making and action items were recorded, and sponsor and manufacturer approvals sought where required.
d. Remote Site Initiation and Activation
Due to the pandemic, on-site visits were not permitted by many NHS Trusts, therefore remote Site Initiation Visits (SIVs) were conducted by our team of CRAs via virtual meetings. We incorporated video links involving medical, pharmacy and laboratory teams to cover critical aspects of the protocol. This could then be used as a training resource for new staff joining the study at any stage. All 18 site SIVs were conducted within a five day timeframe.
All sites required a fast-paced activation process which was separated into four stages, (see diagram below) to allow the sites to undertake certain activities such as advertising and pre-screening/telephone screening activities before booking a vaccine visit in a stepped wise manner.

Gathering, reviewing and Quality Controlling (QC’ing) of documentation throughout the stages was undertaken by the COVBoost CRA, CTA and QA teams. Our clinical study/project manager (CSPM), CRAs and QA team worked collaboratively with the sponsor and the lead site’s (UHS) project team, to develop and release new study specific processes for this aspect.
A SharePoint portal was established for each site, allowing quick and efficient exchange of, and access to, essential documents for the study.
e. Recruitment and Monitoring
As soon as the site contractual process had been completed, expedited delivery of the vaccines were undertaken by the sponsor to ensure the sites were able to commence recruitment activities. Between the 1st June and 30th June 2021, 3498 people were screened. 2878 participants met the eligibility criteria and received one dose of a Covid-19 vaccine or a control.

The Electronic Data Capture (EDC) was reviewed by the COVBoost CRA team as a rolling remote activity using a study-specific EDC tracker which they independently devised and was approved and implemented for this trial
Documentation such as accountability logs, screening and enrolment logs, vaccine shipment logs and temperature records were reviewed centrally by the Covboost CRAs. All participant’s day 28 reactogenicity data had to be monitored, cleaned and analysed to ensure it was submitted to the Joint Committee on Vaccination and Immunisation (JCVI) by August to allow for policy development of the booster vaccination programme.
6. Improved outcomes through collaboration and agile working
From initial feasibility, essential document development, through to approvals (including further amendments), and all site activations took four weeks, an unprecedented delivery time. A study of this nature would normally require at least 6 months for study start up.
Adopting an agile way of working for this trial allowed PHARMExcel and all parties involved to create a responsive and effective performance across the study.
Factors such as virtual comms tools, a rolling review and actions cycle, both remote and central monitoring, and continual evaluation and improvement of processes led the trial to capitalise on change quickly and ensure results were delivered within a very strict deadline.
The sites have now completed recruitment for the main study, and the PHARMExcel team continue to oversee the final stages of the main study with additional sub studies, to ensure regulatory compliance and recruitment targets are met.
Whilst it was certainly a challenge, this real-time example demonstrates what can be achieved through effective sponsor-CRO-site and regulatory collaborations.
If you’re looking for an experienced CRO to deliver your clinical trial on time and on target, contact us today.
Published 11 February 2022
