We’re thrilled to announce our newest paper in Frontline Gastroenterology is now published online.
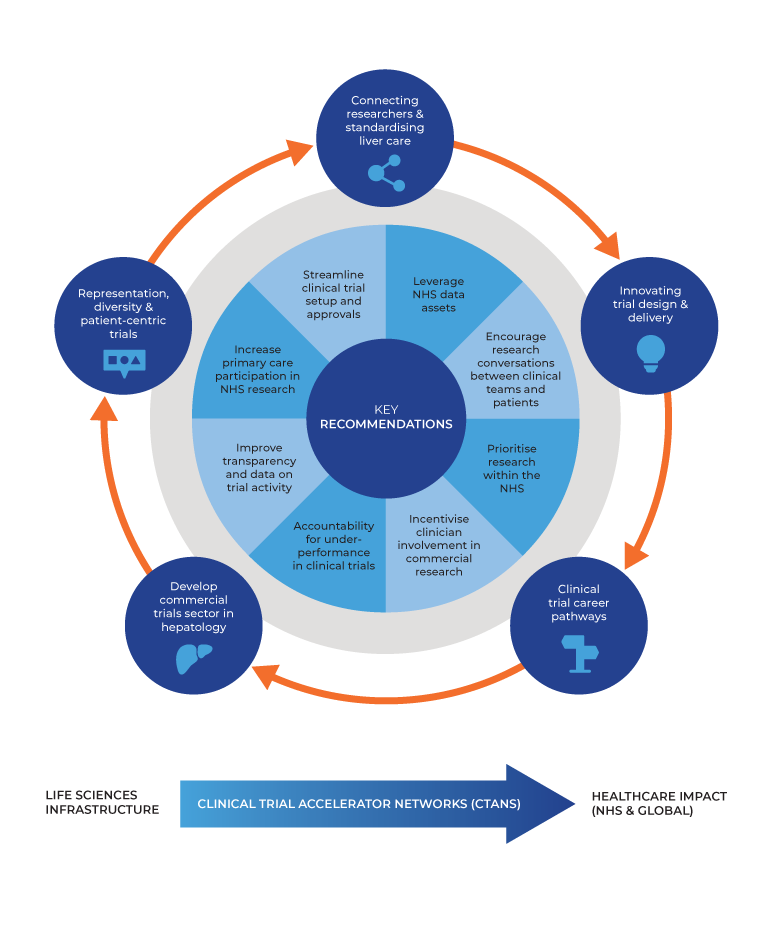
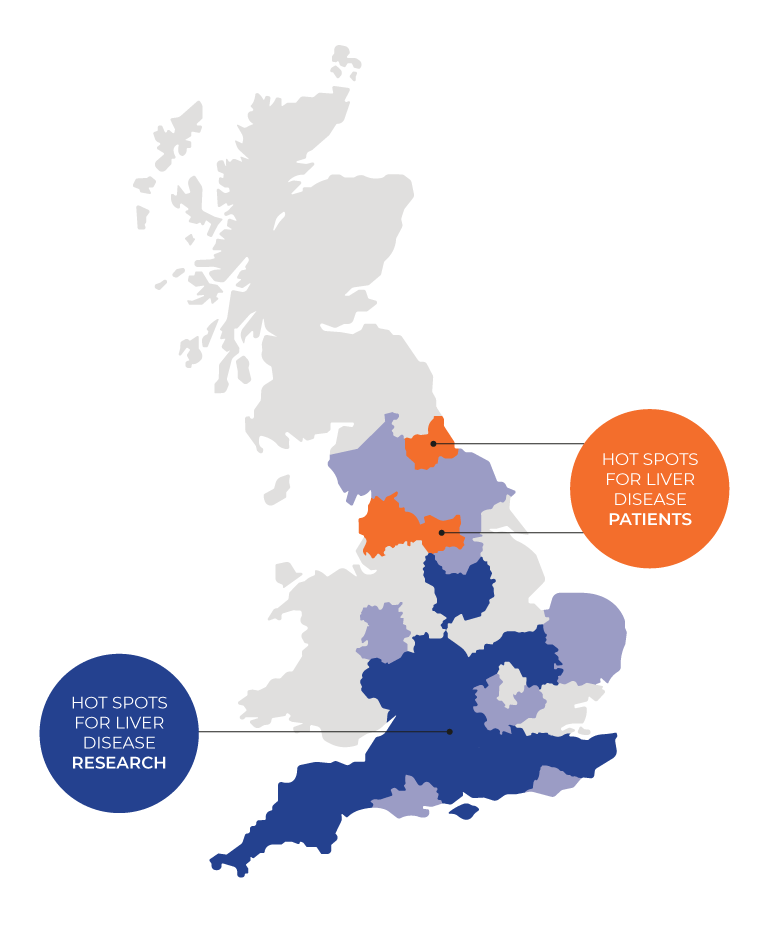
The article “From O’Shaughnessy to Opportunity: Innovating Hepatology Trials in the UK” explores the challenges and opportunities in conducting clinical trials for liver disease, particularly decompensated cirrhosis, within the UK. It reflects on historical achievements, current obstacles, and future directions to enhance clinical trial performance, emphasising the necessity of innovative approaches post-COVID-19 pandemic. The review draws on insights from the O’Shaughnessy report and the UK-Chronic Liver Failure network (UK-CLIF), highlighting key areas for improvement and collaboration.
To cite: Enever Y, Tavabie OD, Green D, et all. Frontline Gastroenterology doi 10.1136/flgastro-2024-102710