PHARMExcel announces publication of its report in the Journal of Hepatology.
Now available online >
Journal of Hepatology: Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on- chronic liver failure
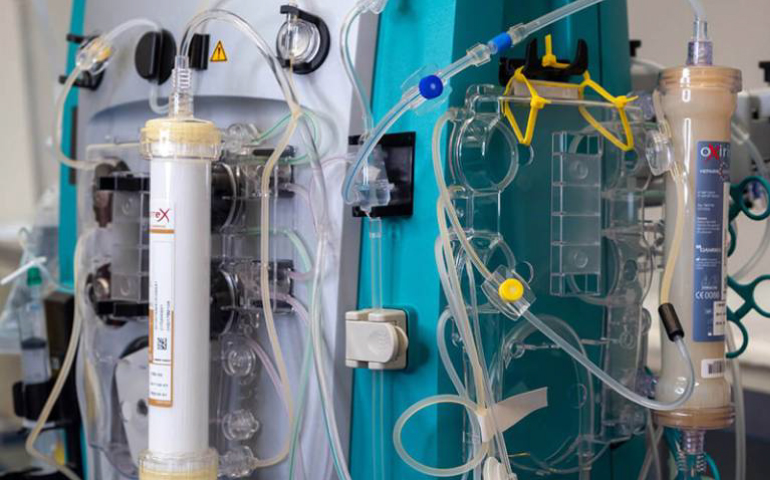
As the UK CRO supporting the pan-European DIALIVE study, PHARMExcel is delighted to announce that together with researchers from UCL, the Royal Free Hospital, UCL spin-out Yaqrit, and their collaborators, it has successfully completed the first in-patient trial of liver dialysis. The trial involved the use of a device called DIALIVE, invented by researchers at UCL’s Institute for Liver and Digestive Health. The device was found to be safe and led to substantial improvement in symptoms and organ function in a great proportion of patients with acute-on-chronic liver failure (ACLF) compared to standard of care. The next step is to conduct a larger clinical trial, and if successful, DIALIVE could be approved for clinical use within the next three years.
ACLF is a condition that causes a sudden decline in liver function, posing a high risk of short-term death. Globally, there are around 100 million people living with cirrhosis of the liver, with about 10 million having cirrhosis along with an additional complication. The UK alone sees approximately 15,000 ACLF patients each year, and their treatment costs the NHS around £100,000 per patient without improving mortality risk.
The liver performs numerous vital functions, including the removal of harmful substances from the blood and the production of albumin, a protein that helps absorb unwanted substances in the body. In ACLF, liver function is seriously impaired, leading to liver cell death and bacterial leakage from the gut into the bloodstream. DIALIVE was designed to address these mechanisms that contribute to mortality in ACLF patients.