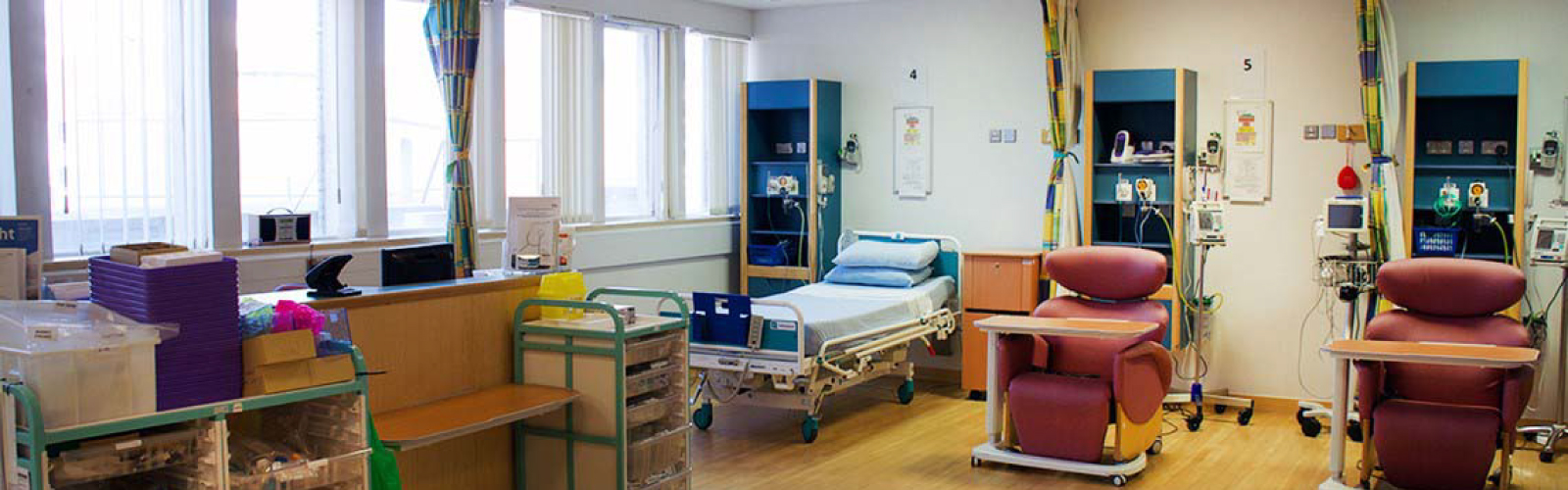
The UK’s Clinical Research Facilities (CRFs) and the departments and expert staff they provide are fundamental in the delivery of high intensity experimental research. By providing a dedicated site for research to take place, patients are more likely to be offered, and in turn, to participate in important clinical trials.
The UK Clinical Research Facility (UKCRF) network supports 54 sites based within NHS trusts in the UK and Ireland, providing centres of excellence, facilitating research, and bringing together NHS, academic, and industry expertise. In 2023, it was awarded £2.4 million funding by the National Institute for Health and Care Research (NIHR) to support research studies for five years.
As a CRO specialising in early phase trials, PHARMExcel works closely with CRFs across a broad portfolio of studies including rare disease such as Behcet’s Syndrome, COVID-19, liver dialysis device, and oncology. Our CRO services range from site selection/feasibility, clinical monitoring, eTMF and CTMS support to study close – always with a focus on participant safety and data integrity. CRF staff also receive training from us, and both parties work together to ensure study compliance throughout.
Unique features of a clinical research facility
CRFs are built to be centres of excellence, hosting the latest technology, and allowing access to expert researchers, all with the shared goal of advancing medical understanding and changing the lives of patients with a wide range of conditions.
By providing an infrastructure for industry led studies, CRFs are responsible for bringing together both NHS and academic expertise with multidisciplinary teams working together efficiently to deliver complex study protocols. With their agile infrastructure and flexible space allowing for them to adapt to meet the diverse needs of the researchers, they provide a safe and quality assured environment.
How a state-of-the-art clinical research facility can support your study
NIHR Liverpool NHS CRF, based in Royal Liverpool University Hospital, is an excellent example of a purpose-built research facility conducting high quality research. It was opened in 2009 and was the first site to achieve the MHRA Phase 1 Accreditation. This means the site has exceeded the basic regulatory good clinical practice (GCP) standards by having additional procedures, including the highest standards for avoiding harm to trial subjects and for handling any medical emergencies.